PAST ISSUES
 Judith Klavins, "Underwater Galaxy (coral reef spawning at night)". mixed media on canvas.
Judith Klavins, "Underwater Galaxy (coral reef spawning at night)". mixed media on canvas. Coral spawning occurs in the summer months - often around the full moon. During a spawning, corals will release sperm and egg into the water to create larvae which then must survive predators and unfavorable currents to eventually settle upon coral reefs where the larvae can latch and grow into maturity.
Coral spawning occurs in the summer months - often around the full moon. During a spawning, corals will release sperm and egg into the water to create larvae which then must survive predators and unfavorable currents to eventually settle upon coral reefs where the larvae can latch and grow into maturity.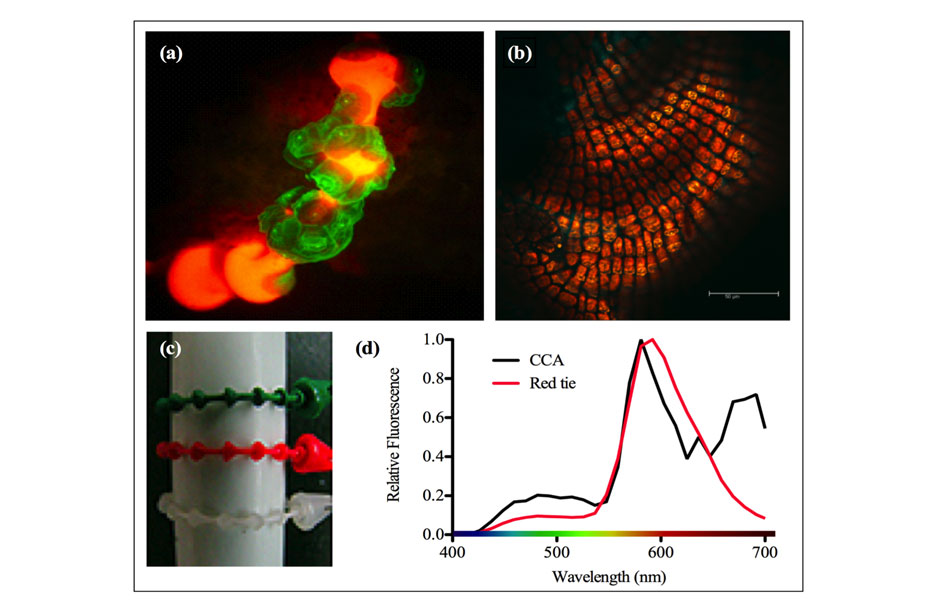 Photos from an experiment conducted by Ben Mason, Ph.D, illustrating how coral larvae find reefs - the most likely place for them to thrive - as they drift in the open sea. Photos 3 and 4 illustrate how the larvae chose to settle on the red plastic zip ties - an unplanned result which led to the discovery that the coral larvae were attracted to the color red - the same color as the algae that grows on corals.
Photos from an experiment conducted by Ben Mason, Ph.D, illustrating how coral larvae find reefs - the most likely place for them to thrive - as they drift in the open sea. Photos 3 and 4 illustrate how the larvae chose to settle on the red plastic zip ties - an unplanned result which led to the discovery that the coral larvae were attracted to the color red - the same color as the algae that grows on corals.
On The Same Wavelength
By Rachel SilversteinOne night a year—no one can predict precisely when—one of nature’s great mating dances occurs. In late summer, when the days are long and the moon is full, the conditions are ripe for a contagious coral spawning spree. Affixed to the ocean floor, corals have to mate by releasing sperm and eggs all at once into the water. They then use the “motion of the ocean” to mix the sperm and eggs together to form baby corals, or “larvae”. Larvae are biologically-basic, featureless life-forms that resemble nearly-microscopic floating papayas with one programmed life goal: swim. As with human romance, the timing of this coral spawning is everything. By flooding the ocean with sperm and eggs, corals can overwhelm hungry mouths and increase the likelihood of having baby corals.
Most coral larvae will be consumed within hours of spawning, victims of the massive feeding frenzy that coral spawning typically triggers. Those larvae that survive, however, are carried by ocean currents to distant regions, where they hope to land on a suitable hard surface — like a reef — attach themselves, and grow into new coral colonies.
But, to appropriate the words of Donald Rumsfeld, “there are known unknowns” in this process, such as, how do these basic, featureless lifeforms, with no apparent way to perceive the world, locate reefs? Choosing the wrong place to attach could mean death for the baby coral.
Scientists have long-realized that coral larvae are attracted to crustose coralline algae, a red encrusting algae that resembles underwater rust because it grows on almost anything left in the ocean. This algae grows on hard, underwater surfaces, often on reefs; so, if larvae can find this algae, they’re likely to find a reef. A few chemical cues had been implicated in the attraction, but no-one has yet been able to identify all the workings of the precise mechanism.
In 2007, Ben Mason, then a Ph.D. student at the University of Miami’s Rosenstiel School of Marine and Atmospheric Science, was trying to understand how coral larvae chose to settle on new reefs. Night after night, he waited on a boat, watching and hoping, diving among jellyfish and other unseen denizens of a pitch-black ocean, to catch corals spawning to collect the larvae. Finally, he saw the big show: millions upon millions of coral sperm and coral eggs were released into the warm, black waters off Key Largo, Florida. He dove into the ocean and began collecting the sperm and eggs, allowing them to mix and form larvae in buckets, which he then placed into different tanks for his experiment. Back at his lab, Ben put different tiles, each representing a different type of the ocean bottom, in a series of tanks, and waited.
Over the next few days, the corals began to settle, and a strange pattern began to emerge. Instead of settling on the tiles, the corals were settling on the plastic zip ties that attached the identifying labels to the tiles. And not just any zip ties. The larvae only settled on the red zip ties. Ben repeated the experiment and, each time, he ended up with the same results. It gradually became clear that these coral larvae, lacking any obvious external organs, were selecting their destinations based on color.
After testing the wavelengths emitted from encrusting algae, Ben found that, by chance, the wavelengths matched almost exactly with those emitted by the red zip ties. Here, Ben had stumbled upon the answer to one of the biggest questions in coral biology. Corals were attracted to this particular color of red, a color that, in nature, is perceived almost identically to encrusting red algae. But, like all good scientific answers, it only led to more questions. How were these corals, lacking organs to observe the world around them, perceiving color?
Through the rest of his Ph.D. work, and now onto his post-doctoral research, Ben found that these primitive beings are actually studded with “opsin” proteins, similar visual proteins to those that are allowing you to read the words on this page. In other words, these coral larvae are probably the most ancient example of vision. Although it was thought that the evolutionary advantage of vision is so profound that it evolved independently as many as 19 times, it is now thought to have a single origin; our vision and that of corals may have evolved from the same common ancestor.
What is likely, however, is that coral larvae evolved to be attuned to the exact biological pulse emitted from the reef in the form of these red wavelengths –a signal drawing the drifting larvae in like a beacon. These algae are key to why corals are able to grow in aggregated ecosystems and form reefs, oases of productivity in what is the desert of the oceans, and the foundation of the most diverse ecosystems on our planet. (Incidentally, climate change and ocean acidification makes it harder for this algae to grow). If we wish to understand the world, one lessons from Ben’s work is the importance of opening our eyes to unexpected results. Biology often takes you between the commas, in sounds that only dogs can hear, and in colors that only eye-less coral spawn can see.
The possibilities of discovery are endless, still.
